This is part of a series dedicated to Digital Therapeutics, by Jovan Stevovic, CEO and founder chino.io
An overview of the main reimbursement frameworks and how to get them done.
A new era for healthcare and digital innovation is coming and will enable healthcare systems to deliver better care and improve the quality of life. In the last 2 years, we all have seen the rise of the digital version of medicines – better known as Digital Therapeutics (DTx).
Investors have seen long-term value in companies developing digital therapeutics in the last three years. Data Bridge forecasted that the European DTx market is going to grow nearly five times to almost $11 billion in 2028 compared to 2021 – with an average annual rate of more than 23%. Germany, the UK, France, and Belgium are set to remain the largest markets in Europe.
DTx are increasingly being recognised and adopted into national frameworks worldwide to improve patient care and quality of life.
Doctors and paid insurers can now prescribe digital health apps, and suddenly, there’s a new business model for digital health applications. However, to benefit from this, digital therapeutics will need to be approved for reimbursement in every single country.
Towards a common definition of Digital Therapeutics?
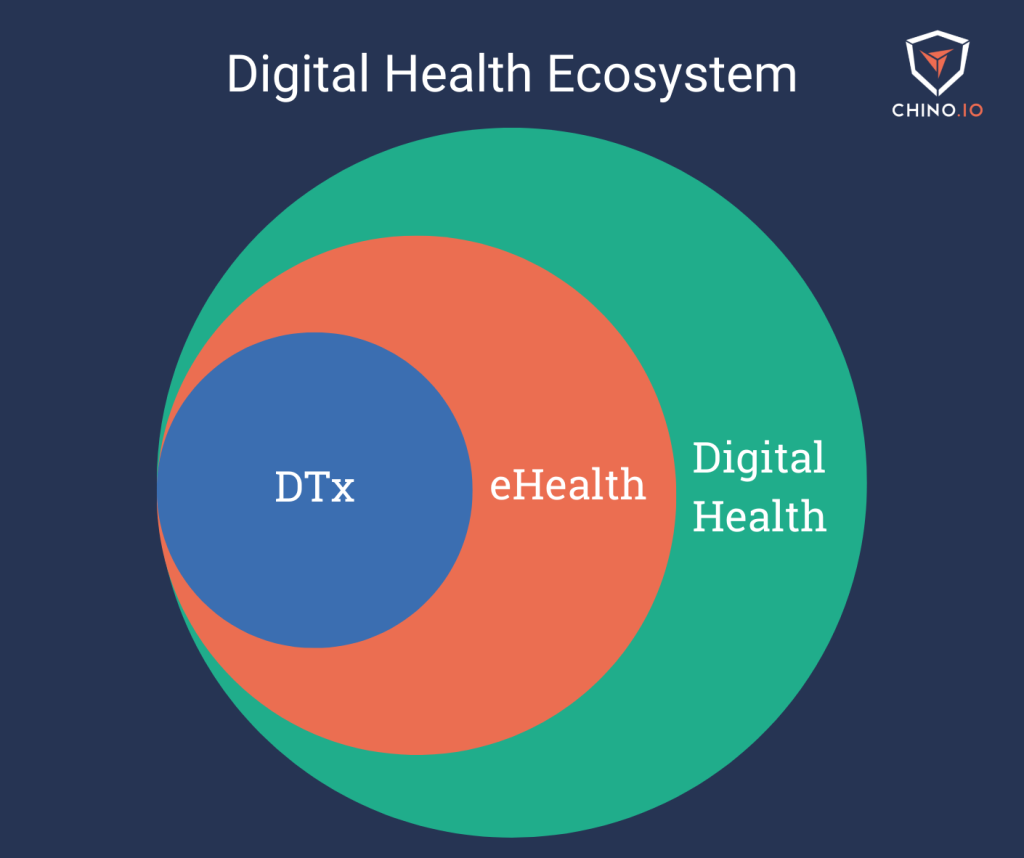
The EU Parliament describes Digital Therapeutics (DTx) as “evidence-based therapeutic interventions driven by software to prevent, manage, or treat a medical disease.”
For this reason, digital therapeutics are regulated as medical devices and categorised by regulatory frameworks as part of the subset of Software as a Medical Device (SaMD).
From a legal point of view, DTx can fit within many different categories, such as eHealth, mHealth or health apps. This is also demonstrated by the current variety of classes DTx can fall under: mHealth for Belgium, DiGAs for Germany, digital health apps and digital health technologies for UK NHS and NICE.